Hello,
Is there a topic in genetics or metabolism that you find challenging or want to learn more about? Send your requests for topics to cover in upcoming newsletters to studyraregenetics@gmail.com (or just reply to this email).
Also, please feel free to forward this newsletter to anyone who might be interested. Thank you to the 90 of you who have subscribed so far!
-Daniel
Questions
Question 13
A 4-year-old boy presents with difficulty walking and fatigue. He started walking at 20 months. He has trouble climbing stairs and cannot walk nearly as far as his 3-year-old brother. Family history is notable for a maternal uncle with progressive muscle weakness who died in his early twenties. Physical exam shows a waddling gait with lumbar lordosis, enlarged calves, and a positive Gower sign. A serum CK level is 8500 U/L, and genetic sequencing reveals a pathogenic multi-exon deletion in DMD. Given the diagnosis, which of the following treatments will slow the loss of motor milestones?
Question 14
During a follow-up counseling session, the patient’s parents state that they are planning to have additional children. Targeted parental testing shows that patient’s multi-exon deletion in DMD is also present in his asymptomatic mother. Which of the following is most correct regarding the mother’s recurrence risk of having a child with this condition?
Explanation
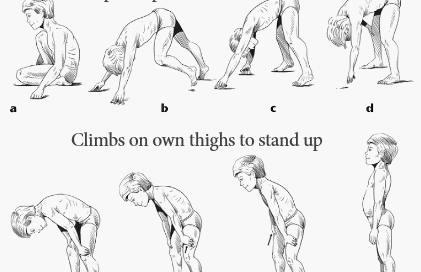
Duchenne muscular dystrophy (DMD) is a disorder characterized by progressive skeletal and cardiac muscle weakness. DMD is the most common muscular dystrophy diagnosed in childhood. Typically, muscle weakness (e.g. fatiguability, difficulty climbing stairs) is the presenting symptom and occurs between 2 and 3 years of age. The weakness affects large proximal muscles (e.g. quadriceps, hip flexors) before affecting more distal muscles. Proximal muscle weakness may manifest as difficulty climbing stairs (as in this patient) or as a positive Gower sign (see image below), whereby a patient seated on the floor pushes on their thighs with their hands and arms to lift their body in an attempt to stand up. Physical exam may also show calf pseudohypertrophy, whereby the calves appear enlarged due to the deposition of fibrofatty tissue rather than muscle hypertrophy.
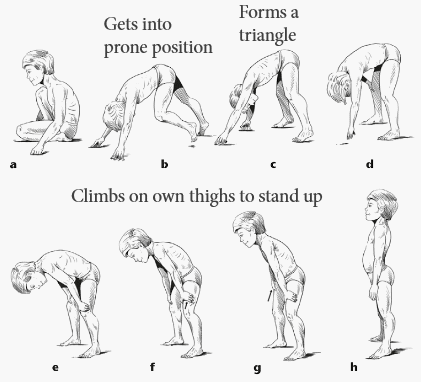
Though differences in gross motor function are usually the presenting symptom, patients with DMD may also develop cardiomyopathy, cardiac conduction abnormalities, low bone mineral density, and scoliosis, particularly as the disease progresses. In addition to delayed motor milestones, some patients also have delayed verbal milestones as part of a non-progressive mild cognitive impairment. Patients with DMD require a wheelchair by the early teenage years and often die in their late teens or early twenties, most commonly from respiratory failure due to diaphragmatic involvement.
Treatment
The first-line treatment of DMD is with glucocorticoids such as prednisone or deflazacort (Question 13). Deflazacort is a steroid similar to prednisone that is associated with a better side effect profile (e.g. less weight gain). These glucocorticoids slow the rate of decline of gross motor and pulmonary function, reduce the risk of scoliosis, and may delay the onset of cardiomyopathy. For patients with certain variants, antisense oligonucleotides (ASOs) that promote exon skipping are also available. For example, casimersen is an ASO available for the ~8% of patients with DMD who have a mutation that is amenable to exon 45 skipping (for reference, DMD has 79 exons in total). These ASOs are delivered via peripheral IV and not intrathecally (Question 13), as the target site of action is the skeletal muscle rather than the central nervous system. Looking forward, multiple ongoing clinical trials are using ASOs, gene replacement, and small molecules in an attempt to treat various facets of this disease.
Genetics and inheritance patterns
DMD is caused by genetic variants in the dystrophin (DMD) gene. The most common type of variant found in DMD are out-of-frame deletions involving 1 or more exons that result in the absence of a functional protein product. In contrast, in-frame deletions in DMD are the most common variant seen in Becker muscular dystrophy, a more mild muscular dystrophy associated with a later onset of symptoms.
DMD is inherited in an X-linked manner, meaning that there is a 25% chance of having an affected son if the mother is a carrier (Question 14), given no prior knowledge about the sex of the fetus. If it is already known that the carrier mother is having a son, then there is a 50% probability that her son is affected with Duchenne. Most boys (~70%) with DMD inherit a variant from their mother, while ~30% have a de novo variant. While the majority of female carriers of DMD mutations are asymptomatic, a small minority may present with muscle pain, muscle cramps, or cardiomyopathy. For symptomatic female DMD carriers, the age of onset and severity of symptoms is highly variable.
Learning objective
Duchenne muscular dystrophy is the most common childhood-onset muscular dystrophy and presents with progressive muscle weakness that eventually results in respiratory failure. Pathogenic variants in the DMD gene are inherited in an X-linked manner, most often from an unaffected carrier mother. Treatment is with glucocorticoids (e.g. prednisone or deflazacort) and with exon-skipping therapy for patients with amenable genetic variants.
2023 ABMGG General Exam Blueprint | V. Single Gene Inheritance → d. Single Gene Disorders (page 3)



Why isn't the answer 25% chance to have an affected son? The possibilities are (1) affected son (2) unaffected son (3) carrier daughter (4) non-carrier daughter, which all have the same probability of occuring.