2022.12.13 | Questions 15-16
A positive newborn screen (elevated C3)
Hello,
Welcome to all the new subscribers from this past week!
I wanted to clarify a point from last week’s newsletter (question #14). The probability of a carrier mother (for Duchenne, an X-linked condition) having an affected son is 25%, given no prior knowledge about the sex of the fetus. If it is known that the carrier mother is having a son, then there is a 50% probability that the son is affected with Duchenne. Based on the way the question was written, the correct answer for question #14 should be 25%. I have updated the explanation from last week’s post to reflect this.
Feedback or comments are always welcome.
Have a great week!
-Daniel
Questions
Question 15
A 1-week-old girl presents to clinic for follow up after her newborn screen identified an isolated elevation of propionylcarnitine (C3). The girl was discharged from the well baby nursery on day of life 2 after an uncomplicated delivery. She is feeding well with no concerns. Follow-up testing reveals low methionine, elevated methylmalonic acid, and elevated homocysteine levels. The patient and her mother both have a normal vitamin B12 levels. Her biochemical testing is most consistent with which of the following disorders?
Question 16
The diagnosis of Cobalamin C deficiency is confirmed on molecular testing. Which of the following treatments should be initiated for this patient?
Explanation
The patient in Question 15 has Cobalamin C deficiency (cblC), a disorder detected on newborn screening that classically presents with elevated methylmalonic acid, elevated homocysteine, and low methionine. CblC deficiency is the most Common disorder of intracellular cobalamin metabolism. The signs and symptoms of cblC deficiency are highly variable and can present in utero, in adulthood, or anywhere in between. CblC deficiency affects several major organ systems including the brain (causing seizures, developmental delay, intellectual disability, and microcephaly), hematologic system (causing renal thrombotic microangiopathy and cytopenias, including megaloblastic anemia), and eye (causing retinal degeneration with maculopathy and vision loss — “Cobalamin C (‘see’) affects your ability to ‘see’”). Patients may also have difficulties with growth that manifest as intrauterine growth restriction (IUGR), difficulty feeding, and slow weight gain. Newborns may be asymptomatic at birth, which is why newborn screening is so important, as it enables early detection and the ability to offer management prior to the onset of symptoms, which generally improves outcomes.
Biochemistry of CblC Deficiency
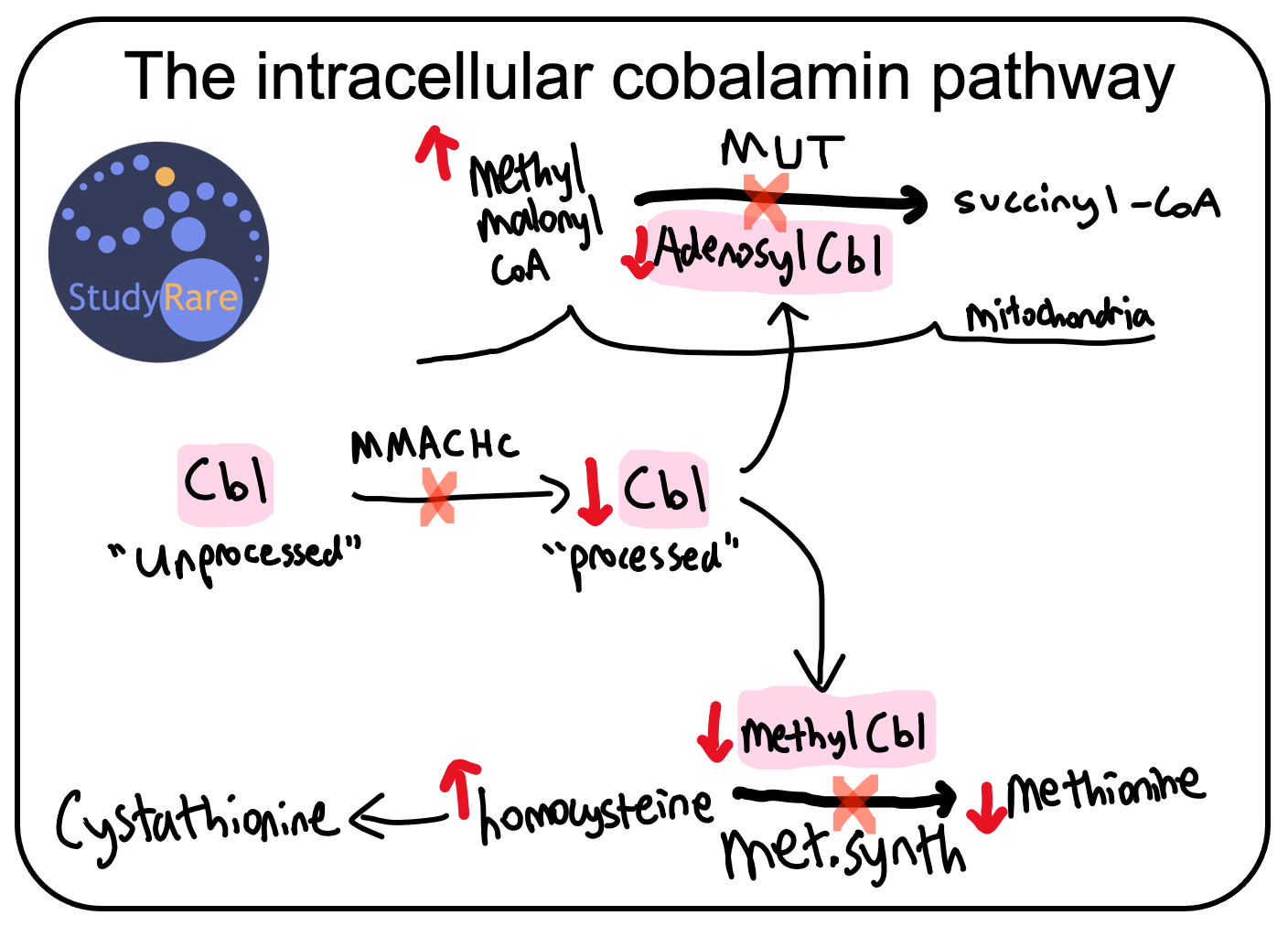
CblC deficiency is caused by mutations in the gene MMACHC, which encodes a protein (MMACHC) that is involved in processing cobalamin, also known as vitamin B12. Cobalamin (cbl) enters the cell in an “unprocessed” state and subsequently undergoes processing by a series of proteins that includes MMACHC (for details, see this diagram). Deficiency of MMACHC results in decreased “processed” cbl, which is normally converted into either adenosylcobalamin, which serves as a cofactor for methylmalonyl-CoA mutase, or methylcobalamin, which serves as a cofactor for methionine synthase (see figure below).
💡Mutations in MMACHC cause MethylMalonic Acidemia Combined w/ HomoCystinuria.
Why the name cblC?
Disorders of intracellular cobalamin metabolism are named based on complementation analysis, a historical approach used in the pre-sequencing era of genetics to identify complementation groups, which are now known today as genes. Each disorder of cobalamin (cbl) metabolism has a capital letter denoting its complementation group (e.g., cblC represents complementation group C). The cobalamin complementation groups corresponding with a disease include cblA - cblJ (excluding cblH and cblI) and cblX.
💡The word cobalamin is a combination of the words “cobalt” and “vitamin”. Cobalamin (aka Vitamin B12) is the only cobalt-containing vitamin.
Management
The goals of long-term management in cblC deficiency include lowering plasma homocysteine and methylmalonic acid and normalizing plasma methionine levels. The mainstay of long-term management is IM or subQ hydroxocobalamin (Question 16), which substitutes as a cofactor for methionine synthase and methylmalonyl-CoA mutase. Though consistent treatment with hydroxocobalamin results in improved biochemical laboratory values, the risk of some complications (e.g. intellectual disability, retinopathy) seen with cblC deficiency is either modestly reduced or unchanged with treatment.
Oral betaine is also used in cblC deficiency and increases methionine levels by activating betaine homocysteine methyltransferase (BHMT). As the name implies, BHMT transfers a methyl group from betaine (aka trimethylglycine) to homocysteine, forming methionine and dimethylglycine in the process. BHMT shares the same reactant (homocysteine) and product (methionine) as methionine synthase, and as such provides an alternative pathway through which to potentially increase methionine and lower homocysteine (see diagram below). In addition, oral folate or folinic acid supplementation can be given to patients with cblC deficiency, as it serves as a cofactor for methionine synthase. Like betaine, folate functions as a methyl group donor.
💡Note that methionine is the methylated version of homocysteine.

In contrast to methylmalonyl-CoA mutase deficiency (due to mutations in MUT), patients with cblC deficiency do not usually experience acute metabolic decompensation in the setting of fasting or illness. For a deeper dive on both acute and chronic management for cblC deficiency and related disorders, check out these guidelines (Huemer et al., 2017).
Incorrect answers
(Question 15) In contrast to cblC deficiency, patients with methylmalonyl-CoA mutase deficiency and propionic acidemia do not have elevations in homocysteine. Patients who present with maternal vitamin B12 deficiency may have biochemical markers similar to cblC deficiency (e.g. elevated homocysteine and elevated methylmalonic acid), however this patient’s (and her mother’s) vitamin B12 levels were both normal, making maternal vitamin B12 deficiency less likely.
(Question 16) Among the answer choices, none of the other management approaches listed besides hydroxocobalamin are routinely given for cblC deficiency. Other strategies such as methionine supplementation and pyridoxine have little evidence of benefit. Supplementation with levocarnitine, a detoxifying agent for organic acids (e.g. methylmalonic acid) and fatty acids, may be given if patients are deficient in carnitine based on blood carnitine levels.
Learning objective
Cobalamin C deficiency is the most common disorder of intracellular cobalamin metabolism. Patients have a broad spectrum of neurologic, developmental, and hematologic symptoms that can present at any age. Laboratory studies show low methionine, elevated methylmalonic acid, and elevated homocysteine levels. Treatment is with hydroxocobalamin, betaine, and folate.
2023 ABMGG General Exam Blueprint | V. Single Gene Inheritance → d. Single Gene Disorders → ix. Metabolic disease (page 3)


Outstanding! Thank you.